Electrolyte Analyzer
Electrolyte analysis equipment is used to detect potassium ions, sodium ions, chloride ions, ionized calcium and lithium ions from samples. The sample can be whole blood, serum, plasma, urine, dialysate, and hydration fluid.

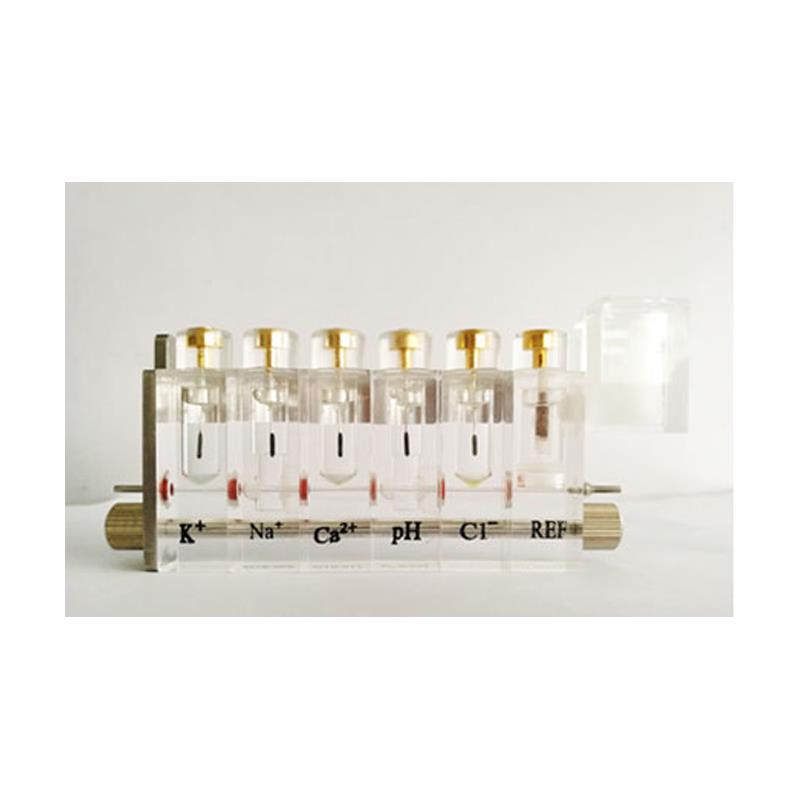

Instrument introduction
a.Instrument use
Electrolyte analyzer is indispensable in clinical testing. In clinical testing, it mainly tests and maintains human blood. The balance of osmotic pressure in body fluids is very important in patients who require large amounts of balanced fluids during surgery, burns, diarrhea, and acute myocardial infarction. The instrument has high precision and accuracy, and the measurement results of any sample are accurate, reliable, fast, and easy to operate. Therefore, ion detection is an indispensable general equipment in hospitals at all levels.
b.working principle
Electrolyte analyzers use ion selective electrode measurement to achieve accurate detection. There are six types of electrodes on the instrument: sodium, potassium, chloride, ionized calcium, lithium and reference electrode. Each electrode has an ion selective membrane that will interact with The corresponding ion in the test sample reacts. The membrane is an ion exchanger, and the membrane potential is changed by reacting with the ion charge. The potential between the liquid, the sample and the membrane can be detected. The two potential differences detected on both sides of the membrane will produce Current, sample, reference electrode, and reference electrode solution constitute one side of the "loop", membrane, internal electrode solution, and internal electrode on the other side.
The difference in ion concentration between the internal electrode solution and the sample will generate an electrochemical voltage on both sides of the working electrode's membrane. The voltage is led to the amplifier through the highly conductive internal electrode, and the reference electrode is also led to the location of the amplifier. The standard solution with known ion concentration obtains a calibration curve to detect the ion concentration in the sample.
When the measured ions in the solution contact the electrode, ion migration occurs in the aqueous layer of the ion selective electrode matrix. The charge of the migrated ions changes and there is a potential, which changes the potential between the membrane surfaces. Between the measuring electrode and the reference electrode Generate a potential difference.
Common electrode structure:
1.Characteristics of sodium electrode: The sodium electrode is a glass capillary electrode used to determine the concentration of sodium ions in a liquid sample. The main structure:
Electrode cover: transparent plastic.
Measuring capillary: Sodium sensitive glass.
Electrode chamber: sealed, filled with sodium electrode solution.
Electrode core: Ag, Agcl
2.Features of Potassium Electrode: Potassium electrode is a kind of membrane electrode, which is also used to measure the potassium ion concentration in the sample.
The main structure:
Electrode cover: transparent plastic.
Measuring capillary: potassium ion sensitive membrane. Electrode chamber: sealed and filled with K+ solution.
Electrode core: Ag/Agcl
3.Chlorine electrode characteristics: The chlorine electrode is also a membrane electrode, used to measure the concentration of Cl ions in the sample.
The main structure:
Electrode cover: transparent plastic.
Measuring capillary: Cl ion sensitive membrane.
Electrode chamber: sealed and filled with Cl- solution.
Electrode core: Ag/Agcl
4.Reference electrode characteristics: The reference electrode is a device that connects the sample and the signal ground.
Main structure: The reference electrode consists of two parts: the reference electrode sleeve and the reference electrode core. The reference liquid in the reference electrode sleeve forms a salt bridge between the reference electrode core and the sample. At the beginning of each measurement, the reference liquid is injected into the reference electrode sleeve and a small part of the reference liquid is made of glass. The capillary infiltrates the measurement chamber to form a salt bridge between the sample and the reference electrode core, and the reference electrode core forms a loop between the electrical signal ground and the reference liquid.
Measurement process
Ion selective electrode, the electrode contains the electrode solution of known ion concentration, through the ion selective electrode membrane and the corresponding ions in the sample interpenetrate, thereby generating membrane potential on both sides of the membrane, the ion concentration in the sample is different, the size of the generated potential signal It is also different. The concentration of ions in the sample can be measured by measuring the magnitude of the potential signal.
The difference in ion concentration between the liquid in the electrode and the sample causes the electrode membrane to generate an electrochemical potential. This potential can be taken out by the electrode and sent to the input of the amplifier. The other input of the amplifier is connected to the reference electrode and grounded. The electrode voltage can be further amplified . The voltage difference formed determines the ion concentration of the tested sample.
research process
When the measured ions in the electrode solution contact the electrode, ion migration occurs in the aqueous layer of the ion selective electrode membrane matrix. There is a potential for the charge change of the migrating ions, which changes the potential between the membrane surfaces; a potential difference is generated between the measuring electrode and the reference electrode. The potential difference of an ideal ion selective electrode to the ions to be measured in the solution should conform to the Nernst equation: E=E0+ log10a(x)
E: Measured potential
E0: Standard electrode potential (constant)
R: Gas constant
T: absolute temperature
Z: ion price
F: Faraday constant
a(x): activity of ion
It can be seen that the measured electrode potential is proportional to the logarithm of the activity of the "X" ion. When the activity coefficient remains constant, the electrode potential is also proportional to the logarithm of the ion concentration (C). The activity or concentration of an ion.
At present, there are many manufacturers that produce sodium potassium chloride ion electrode analyzers, but the electrodes used are basically the same. Sodium is mostly made of lithium aluminum silicate glass electrode film, which has a long life. Potassium electrodes are mostly made of junctional ampicillin film.
The main components of the ion selective electrode analyzer, Na+, K+, and Cl- electrodes, have a specified life span and need to be replaced regularly. Under normal circumstances, if the electrode is maintained for many times, and the pipeline is guaranteed to be unblocked, the electrode that fails to pass after repeated calibration needs to be replaced.
Observing these extremely damaged electrodes, it is found that the reason for the damage is that the electrode liquid level in the electrode is lower than the silver needle surface. When measuring the sample, the measured potential difference cannot be transmitted to the reference electrode through the silver needle for the next step of amplification and determination.
c.Operation method
⒈The instrument is turned on and enters the system self-check to check whether the functions of the main components are normal, such as the main board of the instrument, the printer, the liquid path detection (completed by the liquid detector), the distribution valve and the valve detector, etc., which can intelligently identify and judge the fault, automatically prompt.
⒉Enter the activation electrode program, with electrode activation timing function, accurately grasp the activation time, in order to improve the service life of the electrode and ensure the stability of the electrode. The time is 30 minutes countdown, you can press the NO button to exit the activation electrode program directly.
⒊Enter the main menu, first perform system calibration, which can automatically select base point and slope calibration (you can also choose to exit non-calibration, which is convenient for maintenance and maintenance, but it is not possible to directly measure serum samples)
⒋Select quality control analysis. After more than 5 quality control tests, the quality control report can be automatically generated and printed, and the average value, standard deviation, and coefficient of variation of the number of quality control performed can be calculated.
⒌Intelligent liquid detection program to ensure accurate sampling and measurement, automatic prompts for the measurement process, your convenient guide, 24 hours standby, automatic maintenance in standby mode, automatic positive and negative flushing function, short liquid path, unique positive Automatic backwashing calibration and flushing pipeline system to prevent cross contamination and pipeline blockage.
⒍30 hole position automatic sampling system, with quality control M hole position and waste liquid hole position W position. 30 specimens can be tested at a time.
7. Automatic printing, manual printing are optional, saving printing paper. Report sheet: Comprehensive patient information report, which can be set and printed.
⒏Using American wellhead compression-type power pump tube to increase the service life of the pump tube.
⒐Test samples: whole blood, serum, plasma, urine, body fluid, cerebrospinal fluid, urine, animal serum, etc.
⒑ Measurement method: ion selective electrode (ISE) direct method.
⒒Test items: K+, Na+, Cl-, Ca++, PH, CO2
d.main feature
⒈The function of timing and automatic processing of protein removal solution can remove the adsorption of tubing protein without clogging. The electrode performance is more stable and the test is more accurate.
⒉Side drive automatic reset sampling system, easy to operate, pollution-free, and more environmentally friendly.
⒊The instrument is equipped with a liquid detection program, which can automatically identify and prompt errors in the sampling process, ensuring the accuracy of sampling and measurement.
⒋The electrode is made of imported membrane, with stable performance and long service life.
⒌Automatic potential tracking technology, automatic two-point calibration, double parameter correction of slope and intercept, to ensure the accuracy of test results.
⒍The photoelectric positioning liquid distribution valve has the advantages of high integration, simplified flow path and easy maintenance.
⒎Imported pressure sensor is used to test TCO2, the sensor performance is stable and reliable, and it has obtained national patent protection.
⒏Intelligent maintenance-free design: calibration, sampling, measurement, flushing, display and printing reports, instrument fault diagnosis and troubleshooting, the whole process is automated, without manual cleaning and maintenance.
⒐It can automatically process quality control data, automatically count and print the average, SD and CV values.
⒑ The autosampler is an optional accessory, which can improve work efficiency when there are many samples every day and batch processing is required.
⒒Data can be transferred in batches, test results can be shared in the laboratory through RS-232C standard port or USB2.0 standard port, and database management software is configured to facilitate query and real-time sharing.
e.Precautions
⒈Not all commercially available quality control serums are suitable for the ion electrode method measurement. The quality control serum of some manufacturers contains more additives, which often interferes with the ion method measurement.
⒉Do not inhale air bubbles when the instrument is inhaling the sample, otherwise the result will be unreliable.
⒊This instrument can directly suck samples from the serum separation test tube for analysis, but when sucking samples, be careful not to suck blood clots, so as not to block the pipeline.
⒋If the ambient temperature changes more than 10 degrees, it must be re-calibrated.
⒌The pH value of standard solution and sample should be kept between 6-9, otherwise it will interfere with the determination of sodium content.
⒍Do not use the solution with mildew, turbidity and precipitation. Once the solution is found to be deteriorated, it should be discarded so as not to affect the analysis result.
⒎In combination with clinical response, users should appropriately consider factors that may affect the results, because the use of drugs or internal substances have uncertain conflict effects. Laboratories and clinicians must estimate the results based on the patient's clinical performance.
⒏ Make sure to carry out routine maintenance according to the instructions.
⒐Each electrode is printed with a number. Please take care to protect it. Quality guarantee will not be recognized for any electrode whose number cannot be identified.
f.Safety Precautions
⒈The 220V voltage in the back box of the instrument is dangerous to personal safety. Never open the back cover of the instrument before unplugging the power plug.
⒉Because the samples may contain pathogenic bacteria or viruses, all connecting tubes, pump tubes, electrodes, and waste liquid collection bottles that are replaced by the instrument should be specially treated and discarded.
⒊Operators must disinfect their hands according to professional requirements after completing the operation.
g.Sample collection and processing
⒈Basic precautionary rules must be observed when collecting samples. All blood samples are potentially infectious and may contain human immunodeficiency virus (HIV), hepatitis B virus (HBV) or other terrible pathogens. In order to reduce possible risks in the laboratory, the correct blood collection technique must be mastered. Wearing gloves is essential when handling blood and other body fluids.
⒉For whole blood and plasma, adding the recommended anticoagulant-balancing heparin will not affect the electrolyte value. Sodium heparin is also acceptable, but it restricts ionized calcium, which will cause a decrease in the measurement value range. Other anticoagulants such as EDTA, citrate, oxalate, and fluoride have important effects on blood electrolytes and should not be used. For serum samples, the container does not require additives.
⒊The collection and processing of samples must be completed by professionals. note:
a. Serum and plasma samples stored in the refrigerator can be used for analysis, but they must be returned to normal temperature before analysis.
b. When preparing serum samples, do not add substances that will cause errors in the measurement.
c. The use of tourniquets can cause potassium levels to increase by 10-20%. It is recommended not to use tourniquets when collecting blood, or release the hemostasis after the needle is inserted and two minutes after the needle is pulled out.
d. Because the potassium concentration in red blood cells is much higher than that in extracellular cells, hemolysis must be avoided and separated from the cells as much as possible after collection.
Clinical significance
a.Potassium K
Potassium is the most important cation in the intracellular fluid, which acts as an initial buffer between cells. 90% of potassium ions are in the cells. Damaged cells will release potassium ions into the blood. Potassium is used in nerve conduction, muscle function, and maintains acid-base Balance and osmotic pressure play an important role. High potassium levels appear in oliguria, anemia, dysuria, renal insufficiency caused by nephritis or shock, metabolic or respiratory acidosis, with H ion and K ion exchange Kidney tube acidosis, and hemolysis. Hypokalemia is often an excessive loss of potassium. It is common in: diarrhea or vomiting, insufficient potassium intake, malabsorption, severe burns and increased secretion of aldosterone. The level of potassium will cause Causes muscle stress changes, changes in respiration, and changes in myocardial function. Obtaining potassium values is often used to monitor electrolyte balance in the diagnosis and treatment of the following conditions, such as clinical injections, shock, cardiac or circulatory insufficiency, acid-base balance , Daily treatment, various kidney diseases, diarrhea, excess and deficiency of adrenal cortex function, and other diseases involving electrolyte balance.
[Normal reference value] 3.6-5.5mmol/L.
[Clinical significance]
Increases are seen in renal failure, adrenal hypofunction, shock, tissue crush injury, hypoaldosteronemia, severe hemolysis, oral or injection of potassium-containing fluid, etc.
Reduction is seen in adrenal hyperfunction, severe vomiting, diarrhea, taking diuretics and insulin, barium salt poisoning, metabolic alkalosis, low potassium diet, etc.
b.Sodium Na
Sodium is the most important cation in the extracellular fluid. Its main function to the human body is to maintain osmotic pressure and acid-base balance through chemical action and to transmit nerve impulses. The function of sodium ions is to regulate the potential difference between the inside and outside of the cell membrane to maintain neuronal excitatory conduction Sodium also acts as a factor in some enzyme-catalyzed reactions. The human body has always maintained a basic balance, even if some subtle changes under pathological conditions will be noticed. Low sodium is hyponatremia, which usually reflects excess body fluids relative to the total amount of sodium in the body. Sodium level The decrease in serotonin is related to the following: low sodium influx; sodium loss due to vomiting or diarrhea, supplementation of sufficient water and insufficient salt, improper daily use, or salt-deficient nephropathy; osmotic polyuria, metabolic acidosis; Insufficient adrenal cortex; Congenital adrenal hyperplasia; Dilution due to edema, heart failure, liver insufficiency, hypothyroidism. High sodium value is the loss of water exceeding the loss of salt, such as excessive sweating, excessive breathing, and severe Vomiting or diarrhea, diabetes or diabetic acidosis; aldosteronism, increased renal sodium storage caused by CUSHING synthesis; insufficient water intake due to coma or central disease; dehydration; or excessive alkali therapy. Obtained sodium value is usually used to diagnose Or detect the following: all water balance disorders, clinical injections, vomiting, diarrhea, burns, heart and liver insufficiency, central or nephrogenic diabetes, endocrine disorders and primary or secondary adrenal insufficiency, or other Diseases involving electrolyte balance.
[Normal reference value] 135-145mmol/L.
[Clinical significance]
Increases are seen in anterior pituitary tumors, adrenal hyperfunction, severe dehydration, central diabetes insipidus, excessive input of sodium-containing salt solutions, brain trauma, and cerebrovascular accidents.
The reduction is seen in diabetes, adrenal insufficiency, excessive loss of digestive juice (such as vomiting, diarrhea), severe pyelonephritis, severe renal tubule damage, excessive sweating with diuretics, extensive burns, uremia and polyuria.
c.Chlorine Cl
Chlorine is the most important anion that exists outside the cell. It affects the osmotic pressure of the cell. It also plays an important role in monitoring the acid-base balance and water balance. In metabolic acidosis, when the bicarbonate concentration drops Chloride ion concentration will increase in the opposite direction. Chlorine reduction occurs in severe vomiting, severe diarrhea, ulcerative colitis, pyloric obstruction, severe burns, heatstroke, diabetic acidosis. Addison's disease, fever, and acute infections like pneumonia , Etc. Elevated chlorine occurs in dehydration, Cushing’s syndrome, hyperventilation, convulsions, anemia, cardiac insufficiency, etc. Ionized calcium. Calcium in the blood is free calcium ions (50%) in protein, most of albumin (40%) ) And 10% are limited to anions such as carbonation, phosphate and lactate. However, only ionized calcium can be used in the body for important processes such as muscle contraction, heart function, transmission of nerve impulses and blood clotting. The AVL 9180 analyzer measures the ionized part of total calcium. Diagnoses such as pancreatitis and hyperparathyroidism. Compared with total calcium, ionized calcium is a better indicator.
[Normal reference value]: 96~108mmol/L in chlorine
[Clinical significance]
Increase: excessive intake of chloride, renal failure, oliguria, hyperventilation, alkalosis, urinary tract obstruction, etc.
Reduction: dilutional hyponatremia, long-term use of diuretics, dehydrating agents, severe diabetes, severe vomiting or diarrhea, renal failure and polyuria, etc.
d.Calcium Ca
Hypercalcemia can have a variety of undesirable manifestations, and calcium measurement can be used as a marker by biochemists. Generally, ionized calcium or total calcium has the same effect when detecting malignant tumors, and ionized calcium may be more sensitive .Hypercalcemia often occurs in critical patients with abnormal acid-base regulation and abnormal protein/albumin loss. Calcium can be monitored clearly and effectively by detecting ionized calcium. Nephropathy patients with renal vascular disease usually cause calcium. The concentration of phosphate, albumin, magnesium ion and PH is abnormal. Because these conditions will change the independence of ionized calcium in total calcium, monitoring ionized calcium has become the first choice for accurate monitoring of calcium status in patients with nephropathy. (See Note 3) Ionized calcium It is important for the diagnosis or monitoring of the following: hypertension control, parathyroid, kidney disease, insufficient calcium intake, vitamin monitoring, dialysis patients, cancer, pancreatic disease, diuretic effects, nutritional disorders, kidney stones, multiple myxomas Disease, diabetes, etc.
[Normal reference value]: infant 2.5~3.0mmol/L adult 2.1~2.55mmol/L.
[Clinical significance]
Increased: hyperparathyroidism (hyperplasia, adenoma, adenocarcinoma, tertiary), multiple myeloma, acute osteolytic lesions, bone tumors, uremia, Addison’s disease, Cushing’s syndrome, acral Hypertrophy, vitamin D poisoning, non-osteolytic malignancies, idiopathic hypercalcemia, etc.
Ionized calcium
[Normal reference value]: cord blood 1.30~1.46mmol/L whole blood newborn 1.08~1.18mmol/L; adult 1.12~1.23mmol/L; >60 years old 1.13~1.30mmol/L
[Clinical significance]
Increased: hyperparathyroidism (hyperplasia, adenoma, adenocarcinoma, tertiary), multiple myeloma, acute osteolytic lesions, bone tumors, uremia, Addison’s disease, Cushing’s syndrome, acral Hypertrophy, vitamin D poisoning, non-osteolytic malignancies, idiopathic hypercalcemia, etc.
Decrease: decreased parathyroid function, vitamin D deficiency, infant tetany, renal failure, seborrheic dysentery, acute pancreatitis, obstructive jaundice, long-term fasting, intravenous hypernutrition therapy, etc.
e.Urine electrolytes
Electrolytes exist in the human body and are also taken in from daily food and excreted into the urine through the renal system. This is a natural circulation. The detection of electrolytes in excrement and urine can help understand the condition of the kidneys and other pathological conditions. Information. The test can be quantitatively tested from any urine sample, or from a urine sample collected 24 hours. The amount of electrolyte excreted every day can be obtained by measuring the increase in the excretion concentration of urine in one day (mmol/L).
f.Dialysis electrolysis
In the dialyzer, arterial blood and dialysate are dialyzed on both sides of the dialysis membrane. The dialysis membrane prevents the diffusion of proteins and red blood cells. Because the composition of blood and dialysate is different, a pressure gradient will be generated on both sides of the membrane, and small molecules can pass through the membrane. Diffuse. This method can effectively filter out urea, uric acid and other substances that cannot be excreted due to low renal function. When the electrolyte concentration in blood and dialysate is significantly different, the electrolyte will be processed from high concentration to low concentration. Diffusion, as from the blood to the dialysate, or vice versa.)
Dialysis of electrolytes in dialysis is of great significance to clinicians, such as:
* In order to maintain the electrolyte balance before, during and after dialysis, the deviation can be identified in time, and it can also be corrected early.
* Control the concentration of electrolyte in the dialysate. Generally, a quantitative amount of distilled water and a substance of appropriate concentration are used.








